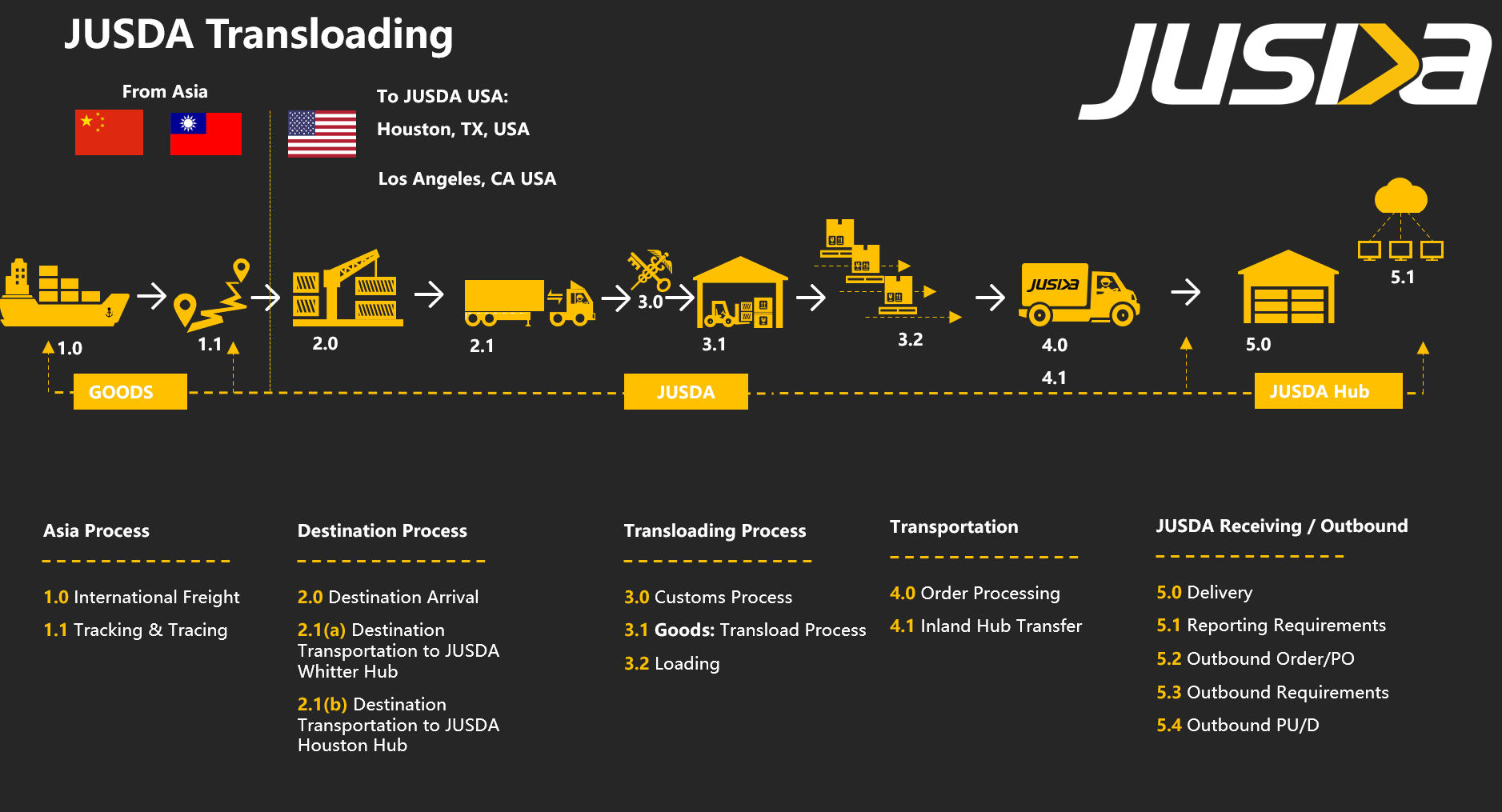
Customs, Duties & Tariffs
COVID-19 PPE Import Resources
Nothing looks the same as everyone navigates the new normal. This is particularly true for importers of personal protective equipment (PPE) products. Imports of healthcare materials and devices must meet strict CBP and FDA requirements prior to release into the U.S. As the need for PPE items has increased, the FDA has loosened restrictions to facilitate the importation of these crucial products. Below, JUSDA has provided a list of resources to keep current with the changing landscape.
- CBP
- COVID-19 Relief Imports Cargo Resolution Team (CCRT) Interactive web portal for COVID-19 inquiries
-
- FAQs
- Submission of inquiries to the cargo resolution team
- CBP COVID-19 Updates & Announcements Customs and Border Protection’s most recent trade related information and CSMS messages
- CSMS messages (ie.# 42124872, #42168200)
- Federal Register Notices
- Trade program updates
- Cargo Security
- PGA codes related COVID 19 Resource table for PGA and HTS codes for importing medical devices and supplies directly related to COVID-19
- Harmonized Tariff Schedule (HTS)
- Partner Government Agency (PGA)
- FDA
- COVID-19 latest guidance
- FDA enforcement guidance Full list of documents are available through this link. Below are a few list of guidance document that have been issued.
- General Face Masks for Medical Use
- Surgical Masks
- Other PPE (gowns, gloves, other apparel, etc.)
- COVID Test Kits
- Hand Sanitizer
- Thermometers
- Infusion Pumps and Accessories
- Emergency Use Authorizations (EUA)
Product specific information on Emergency Use Authorization for COVID-19.
- Personal Protective Equipment EUAs
- In Vitro Diagnostics EUAs
- Ventilators and Other Medical Device EUAs
- Non-FDA regulated products
Personal protective equipment for general purpose or industrial use (that is, products that are not intended for use to prevent disease or illness) is not regulated by FDA.
This information is also noted on CSMS# 42124872.
- WCO & WHO
On April 9th, the World Customs Organization (WCO), in conjunction with the World Health Organization (WHO), issued an HS classification list for COVID 19 medical supplies in response to the current global pandemic. An updated edition was expanded by WHO to include a greater range of medical equipment in addition to WCO’s HS classification reference for COVID-19 medical supplies published a few weeks earlier.
The WCO and WHO in collaboration produced a list of priority medicines for COVID-19, along with suggested HS codes.
Though the FDA has relaxed some restrictions for critical PPE products, this does not mean all requirements have been removed. It is important for importers to conduct due diligence prior to importation to avoid delays of this crucial product.
- The JUSDA Compliance Team